The moment the coronavirus started spreading across the globe, the Society for Assisted Reproductive Technology (SART) and the American Society of Reproductive Medicine (ASRM) promptly released an advisory to infertility patients concerned with COVID-19 (novel coronavirus). The recommendations included precautionary measures for patients/ couples undergoing active infertility treatment and workers in various ART facilities/ laboratories.
Other than COVID concerns, patients who wish to undergo in-vitro fertilization (IVF) are informed to generally undergo screening for infectious organisms (and not only because the coronavirus is a current pressing health issue). This is done due to concerns regarding contamination and the possibility of infection transmission. Contagious organisms like Chlamydia trachomatis, HBV, or HCV in infected couples wanting to go through IVF, may pose a risk to the embryologist and other IVF clinicians. While IVF is not contraindicated in such cases, the embryology clinic and the IVF system warrants further consideration.
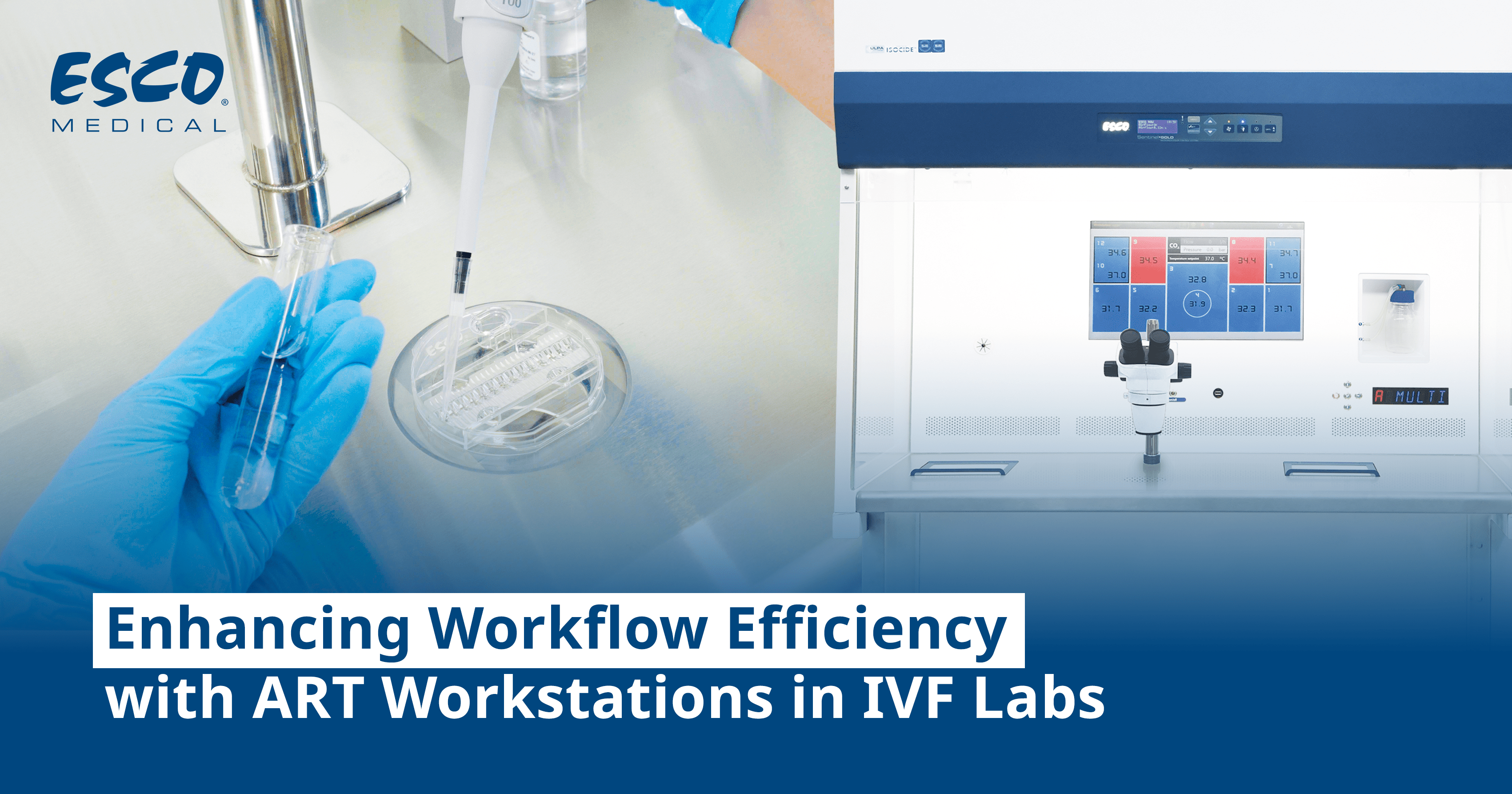
The handling of infectious disease screening of fertility patients necessitates the use of sterile technique and supplementing culture media with screened sera or serum substitutes and antibiotics, and working in an environment that is capable of providing added protection. The transmission of such diseases to IVF laboratory personnel can be further prevented by observing safety guidelines, wearing protective clothing, having proper vaccination (like HBV), developing a plan for the disposal of bio-hazardous materials within the lab, and the use of appropriate engineering controls like biosafety cabinets or ART workstations.
The Esco Medical Class II Workstation can be utilized in handling gametes and embryos from patients with communicable diseases while providing a sample, user, and environmental protection. It has multiple heating zones that enable precise temperature control across the work surface, with the provision of a built-in microscope and integrated MIRI® chambers. (For more information about the MAW Class, check out its brochure at https://www.esco-medical.com/product/ivf-workstation-ii/12/)
IVF laboratory personnel (whether lab technicians or healthcare personnel) are at risk, and so are future mothers and their offspring. Therefore, the safety of all people involved should always be the topmost priority. Fertility clinics should be capable of minimizing these risks by adhering to guidelines and standard operating procedures. Esco Medical is one with the world in reminding everyone (especially reproductive health care professionals and their patients) to stay well-informed of the newest guidelines and updates issued by the Centers for Disease Control and Prevention (CDC) concerning evolving developments on the COVID-19 epidemic.
Want to know more about Esco Medical’s line of workstations and biosafety cabinets? Visit https://www.esco-medical.com/product-directory/

Multi-Zone ART Workstation Class II
Features:
-
Class II protection Multi-Zone heating system with four (4) temperature modes
-
Choice of 4 temperature modes
-
Microscope integration provision
-
Less energy, more savings
-
Touchscreen monitor
-
MIRI® integration
-
Equipped with LED lamp and H14 filter