MIRI® M Multiroom IVF Incubator
The Esco Medical MIRI® M Multiroom IVF Incubator is a modular system with up to 18 independent and movable chambers. Each chamber is designed to maintain a stable and secure environment even during movement, ensuring optimal protection for samples at all times. Its recirculating gas system, with a VOC/HEPA filter and UVC light, maintains optimal conditions, while precise temperature control enhances sample safety. The display provides visual patient confirmation, and built-in software ensures digital verification and tracking, making it a reliable choice for modern IVF laboratories.
Features
Get a Quote Download CatalogueEngineered for ultimate mobility and precision, the MIRI® M Chambers keep embryos in their ideal environment for extended periods, ensuring optimal conditions and more accurate results. Unlike conventional incubators that require frequent sample relocation, our innovative design preserves stability and integrity exactly where it's needed. This advanced approach minimizes environmental disruptions for embryos, which supports better IVF outcomes
The MIRI® M Chambers create an ideal environment for embryos, ensuring they develop optimally. Unlike traditional incubators that need constant sample movement, this innovative design minimizes temperature and gas fluctuations, maintaining stable culture conditions. As a result, enhanced accuracy, improved outcomes, and greater success rates in IVF procedures can be expected.
The MIRI® M Docking Station improves workflow by accommodating up to 18 separate chambers, ensuring efficient organization and easy access. Its scalable design allows labs to manage multiple samples simultaneously while optimizing the vital space inside the laboratory.
*Additional MIRI® M Chambers can be ordered as needed, supporting an undisturbed workflow and greater flexibility in your lab operations.
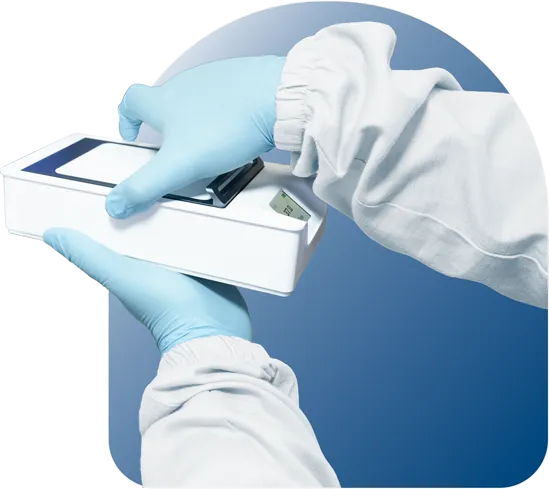
Thoughtfully crafted with an ergonomic surface, MIRI® M Chambers offer a firm grip for enhanced control and stability. This innovative design minimizes handling risks, ensures sample integrity, and empowers users with the confidence they need for successful handling procedures.
The MIRI® M Chambers guarantee accurate patient identification and traceability through a clear graphical display that prominently presents patient information. This built-in safeguard reduces errors, streamlines workflow, and enhances sample security throughout the treatment process. By continuously assigning each chamber to the patient, users can freely change the chamber's position on the docking station without compromising traceability.
MIRI® M multiroom incubator provides information about alarm conditions through expansive pop-ups, icons and colors on the Docking Station‘s and Chambers‘ screens, as well as, backlights in the back panel.
| Technical Specifications | MIRI® M Docking Station |
|---|---|
| Overall dimensions (W x D x H) | 806 × 670 × 643 mm (29.1 x 22.6 x 8.5”) |
| Weight | 85.6 kg (188.7 lbs) |
| Material | Mild steel / Aluminum / PET / Stainless steel |
| Power supply | 115-230VAC, 50-60Hz, 1.2 kW |
| Temperature Range | 35.0 - 39.0 °C |
| Temperature deviation from the setpoint | ± 0.1 °C |
|
Gas Consumption (CO2)
System stable (setpoint reached) - 6 chambers docked System stable (setpoint reached) - 18 chambers docked |
< 0.3 litre/hour < 0.5 litre/hour |
|
Gas Consumption (N2)
System stable (setpoint reached) - 6 chambers docked System stable (setpoint reached) - 18 chambers docked |
< 3.0 litre/hour < 5.0 litre/hour |
| CO2 Control Range | 3.0 - 12.0% |
| O2 Control Range | 3.0 - 10.0% |
| CO2 and O2 concentration deviation from the setpoint | ± 0.2% |
| CO2 Gas Pressure (input) | 0.7 – 1.5 bar (10.15 – 21.76 PSI) |
| N2 Gas Pressure (input) | 0.7 – 1.5 bar (10.15 – 21.76 PSI) |
| Operating altitude | Up to 2000 meters (6560 feet or 80kPa – 106kPa) |
| Shipping weight | 122.3 kg (269.6 lbs) (including the pallet's weight) |
| Shipping dimension | 920 x 800 x 850 mm (36.2 x 31.5 x 33.5") (device on the pallet) |
| Technical Specifications | MIRI® M Chamber |
|---|---|
| Overall dimensions (W x D x H) | 113 x 215 x 59 mm (4.5 x 8.5 x 2.3”) |
| Net Weight | 1.03 kg (2.2 lbs) |
| Material | Aluminum / PET / Stainless steel |
| Rated power input | 24V DC, 1.58A, 38W |
| Battery | 3.6V, 12.42 Wh, Li-Ion |
| Undocked chamber battery time | 30 minutes (without the lid opening) |
| Shipping weight | 1.3 kg (2.9 lbs) |
| Shipping dimension | 122 x 324 x 163 mm (4.8 x 12.8 x 6.4") (when sent separate) |
| MIRI® M Multiroom Incubator | ||
|---|---|---|
| Item Code | Model Code | Description |
| 2074001 | MRI-M-DS6C-8/9 | MIRI® M Multiroom IVF Incubator (Docking Station + 6 Chambers) |
| 2074002 | MRI-M-DS12C-8/9 | MIRI® M Multiroom IVF Incubator (Docking Station + 12 Chambers) |
| 2074003 | MRI-M-DS18C-8/9 | MIRI® M Multiroom IVF Incubator (Docking Station + 18 Chambers) |
| 2074006 | MRI-M-1C | MIRI® M Chamber (MIRI® M-C) |
| 2074004 | MRI-M-6C | MIRI® M Chamber (Pack of 6pcs; MIRI® M-C) |
| 2074005 | MRI-M-12C | MIRI® M Chamber (Pack of 12pcs; MIRI® M-C) |
| Accessories | ||
|---|---|---|
| Item Code | Model Code | Description |
| 1320552 | MRI-M-VOC | MIRI® M VOC/HEPA Filter |
| 1320553 | MRI-M-FD | MIRI® M Heating Optimization Plate for Falcon® dishes |
| 1320554 | MRI-M-ND | MIRI® M Heating Optimization Plate for NuncTM dishes |
| 1320555 | MRI-M-BD | MIRI® M Heating Optimization Plate for BIRR dishes |
| 1320556 | MRI-M-PD | MIRI® M Heating Optimization Plate without footprint for Plain dishes |