MIRI® II-12 Multiroom Incubator
The Esco Medical MIRI® II-12 Multiroom Incubator is intended to be used to provide a stable culture environment at or near body temperature and CO2 and N2 gasses for the development of gametes and embryos during In Vitro Fertilization (IVF) and Assisted Reproduction Technology (ART) treatments.
Features
Get a Quote Download Catalogue Watch Video
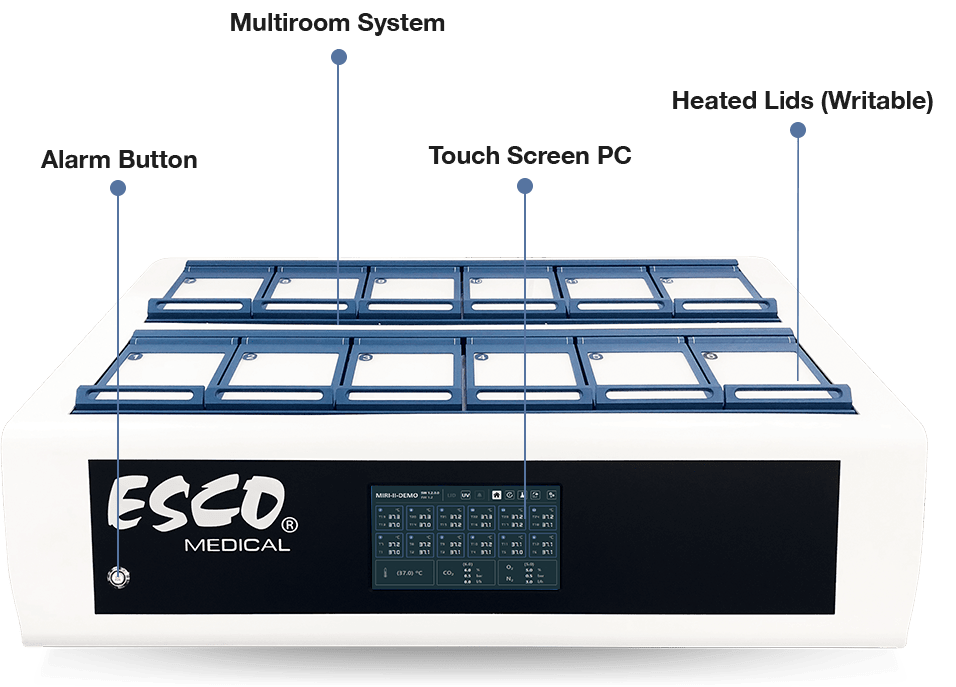
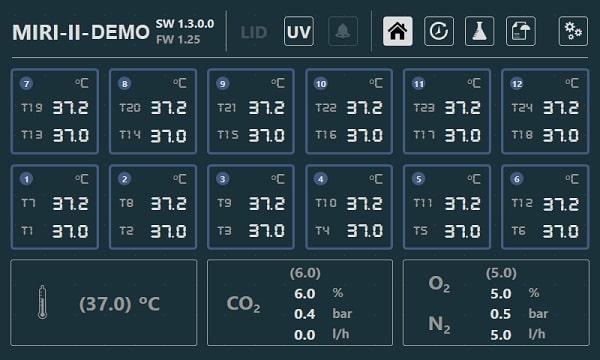
To ensure maximum performance and an environment with the least amount of stress for embryos, the Esco MIRI® II-12 Multiroom Incubator dedicates one chamber for each patient and has 24 completely separate PID temperature controllers.
The chambers are separate from one another and do not affect each other's temperatures in any way. There is no effect on the remainder of the system from any disruption in one chamber, such as a temperature reduction after opening the lid. The temperature in the culture chambers and the lids can be controlled and regulated as well. The top and the bottom of each chambers are separated with a PET layer so that the lid temperature would not affect the bottom.
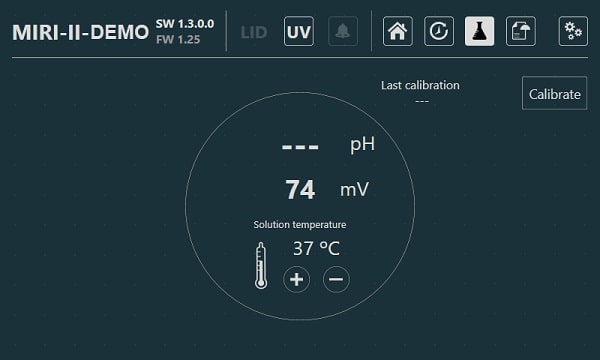
For validation purposes, each compartment has a PT-1000 sensor built-in. The circuitry is separated from the unit's electronics, so it remains a truly separate validation system.
The incubator needs 100% CO2 and 100% N2 to control the CO2 and O2 concentrations in the culture chambers. A dual-beam infra-red CO2 sensor with extremely low drift rates controls the CO2 levels. On the other hand, a chemical, medical-grade oxygen sensor controls the levels of the O2 in the system.
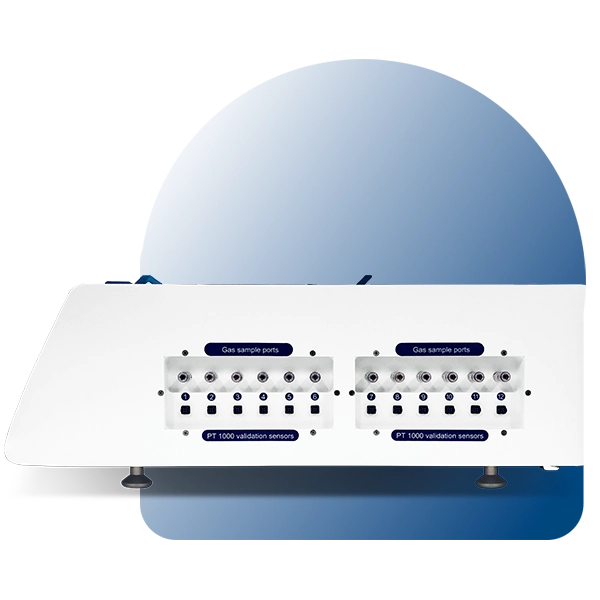
CO2 recovery takes an average of 3 minutes after opening the lid.* The MIRI® II-12 incubator is fitted with 12 gas sample ports that allow the user to validate gas concentration by sampling gas from the individual compartment.
The incubator also features a recirculated gas system where gas is continuously put into the compartment and taken out at the same rate. Gas is cleaned via a 254 nm UVC light with direct gas contact between the bulb and gas. The UVC light has filters that inhibit any 185 nm radiation that would produce dangerous ozone. The filters are located under the UVC light. The complete gas repletion in the system takes less than 5 minutes.
*If the lid has not been open for more than 30 seconds (based on internal testing; performance may vary depending on various factors and environmental conditions)
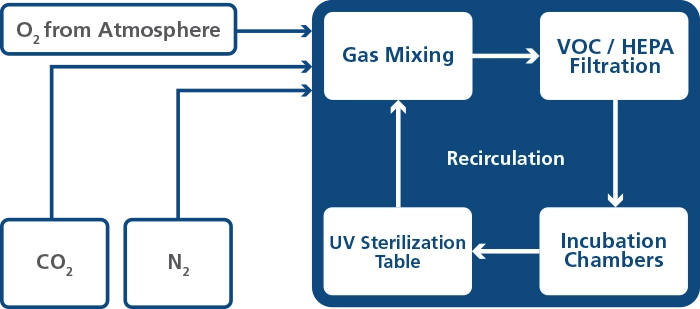
Prevents condensation, enhances consistency throughout each chamber, and improves temperature regulation and recovery.
Allows for reliable temperature regulation by providing direct heat transfer to the culture dishes.
The quick recovery of temperature and gas characteristics after opening a chamber is a significant advantage when utilizing the MIRI® II-12 multiroom incubator.
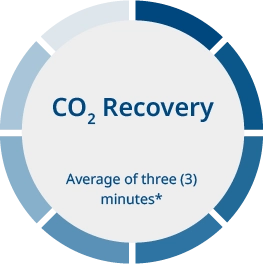
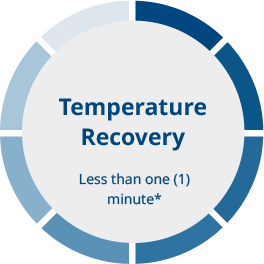
*If the lid has not been open for more than 30 seconds (based on internal testing; performance may vary depending on various factors and environmental conditions)
For gas and temperature validation, the MIRI® II-12 can be connected to an external device such as the Esco MIRI® GA. The primary circuitry is fully separate from the built-in PT1000 temperature sensors. Additionally, all 6 or 12 chambers have gas sampling ports.
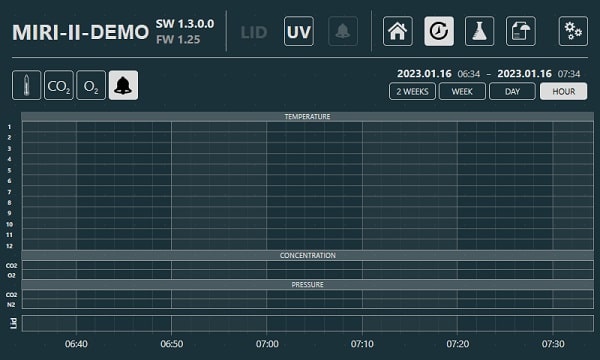
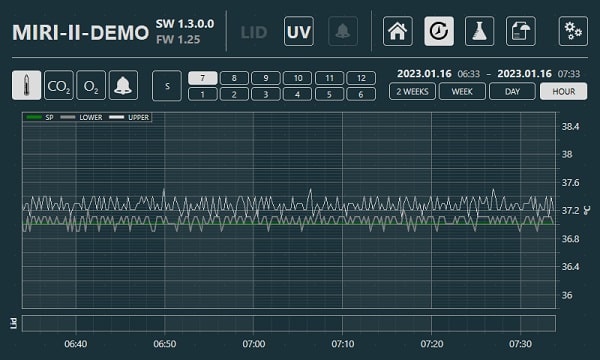
One computer can control and connect to multiple machines. The machine's real-time parameters can all be simply viewed in the MIRI® II-12’s built-in touchscreen PC. These parameters include every temperature and gas concentration points, as well as the set points for all gas input pressures and flow rates. Graphs are also available for viewing that show all machine performance information, including alerts, which are continuously documented. Weekly reports are also generated automatically by the data logger, making it more user-friendly.
A heating optimization plate is included in each chamber to help heat transfer directly to the culture dishes. Each chamber contains inserts that suit different-sized dishes.
| Overall dimensions (W x D x H) | 740 x 575 x 215 mm (29.1 x 22.6 x 8.5”) |
| Net Weight | 47 kg (99.2 lbs) |
| Material | Mild steel / Aluminum / PET / Stainless steel |
| Power supply | 115V 60Hz or 230V 50Hz |
| Power consumption | 500W |
| Temperature Control Range | 25.0 - 40.0 °C |
| *CO2 Gas Consumption | < 2 liters per hour |
| **N2 Gas Consumption | < 12 liters per hour |
| CO2 Control Range | 3.0% - 10.0% |
| O2 Control Range | 5.0% - 10.0% |
| Gas Input Pressure | 0.4 - 0.6 bar (5.80 - 8.70 PSI) |
| Operating Altitude | Up to 2000 meters (6560 feet or 80kPa - 106kPa) |
| Shipping weight | 57 kg (121.3 lbs) (Including the pallet’s weight) |
| Shipping dimension | 890 x 710 x 480 mm (35 x 28 x 18.9”) (device on the pallet) |
* Under normal condition (CO2 set point reached at 6.0%, all lids closed)
** Under normal condition (O2 set point reached at 5.0%, all lids closed)
Increase your
workspace efficiency and maximize space in your IVF laboratory by utilizing the
specially crafted MIRI® II-12 Stacking Frames
MRA2-1014 - MIRI® II-12 Stacking Frame for 2 devices
MRA2-DRAW - MIRI® II-12 Stacking Frame for 2 devices with a drawer
| Stacking frame model | Dimensions with devices affixed (W x D x H) |
|---|---|
| MIRI® II-12 Stacking Frame for 2 devices | 785 x 599.5 x 798 mm (30.9 x 23.6 x 31.4") |
| MIRI® II-12 Stacking Frame for 2 devices with a drawer | 762 x 784 x 580 mm (30.0 x 30.9 x 22.8") |
| On full opening of the drawer: 762 x 1235 x 580 mm (30.0 x 48.6 x 22.8") |
| MIRI® II-12 Multiroom Incubator | ||
|---|---|---|
| Item Code | Model Code | Description |
| Devices | ||
| 2070164 | MRI2-12C-8 | MIRI® II-12 Multiroom Incubator, 230V, 50/60Hz |
| 2070165 | MRI2-12C-9 | MIRI® II-12 Multiroom Incubator, 115V, 50/60Hz |
| Accessories | ||
| 1320011 | MRA-1007 | VOC/HEPA filter (recommended to be replaced every 3 months) |
| 1320498 | MRA2-1014 | MIRI® II-12 Stacking frame for 2 devices |
| 1320499 | MRA2-DRAW | MIRI® II-12 Stacking frame with a drawer for 2 devices |
| 1320045 | MRI-GA | MIRI® GA CO2 / O2 & Temperature Validation Unit, 115V / 230V |
| Heating optimization plates | ||
|---|---|---|
| Item Code | Model Code | Description |
| 1320429 | MRA2-FD | Heating optimization plate for Falcon® Dishes |
| 1320430 | MRA2-ND | Heating optimization plate for NUNC™ Dishes |
| 1320431 | MRA2-VD | Heating optimization plate for Vitrolife® Dishes |
| 1320433 | MRA2-LD | Heating optimization plate for LifeGlobal® GPS Dishes |
| 1320436 | MRA2-OD | Heating optimization plate for SparMED Oosafe® |
| 1320434 | MRA2-PD | Heating optimization plate without footprint for Plain Dishes |
| 1320505 | MRA2-BIRR | Heating optimization plate for BIRR Dishes |