Introduction
IVF incubators are essential to the fields of in-vitro fertilization (IVF) and assisted reproductive technologies (ART). These incubators are used for a variety of purposes, such as maintaining a favorable environment for gametes and cultivating and developing embryos. Traditional incubators like benchtop and box-type incubators feature robust parameter stability and recovery but at the time of a procedure or for embryo check, the embryos are needed to be removed and relocated from the incubator, increasing exposure to the outside. To overcome these issues, portable incubators like the MIRI® M have been designed.
Portable Incubators
Portable incubators are incubation chambers designed to maintain stable temperature, gas composition, while enabling safe transport and short-term culture of gametes and embryos outside the main laboratory setting. The chambers can be connected to a power source or docking station to replenish the chamber charge and other critical parameters.
Portable incubators allow transport of embryos from one place to another for more dynamicity in workflow while ensuring safety from the external environment. This is especially important in hospitals and clinics where the embryologist, along with the embryos, may need to walk a certain distance to reach the operation theatre.
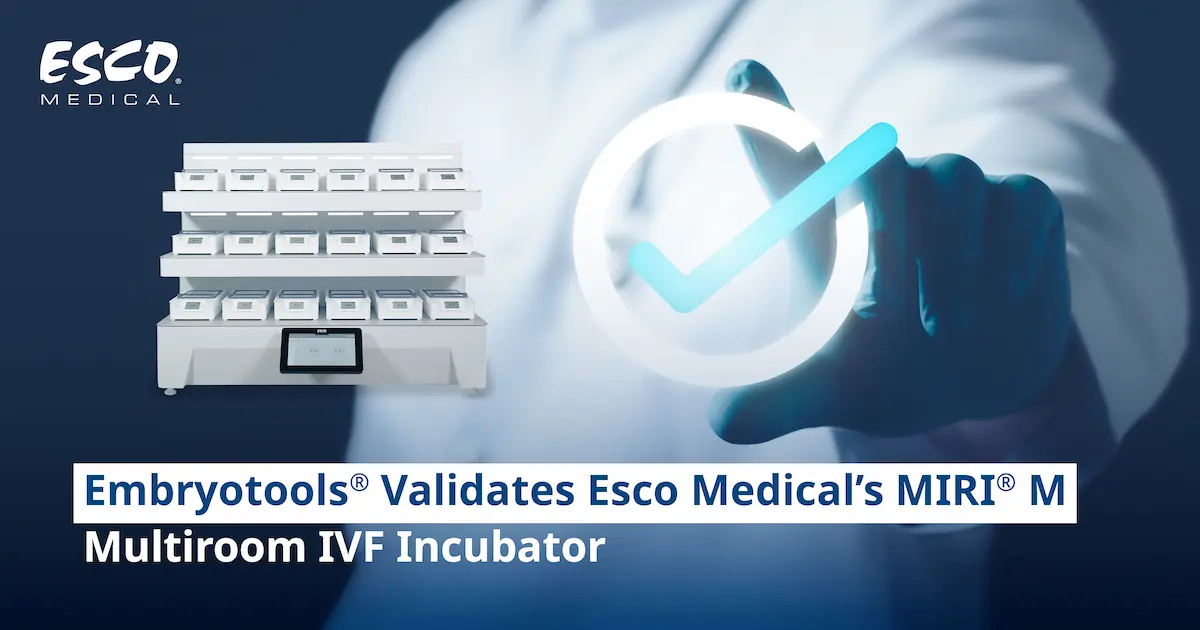
The Esco Medical MIRI® M multiroom IVF incubator is a modular system consisting of a docking station and movable chambers. The incubator can have up to 18 chambers, each being movable between the MIRI® M docking station. The multiroom IVF incubator features a recirculating gas system where gas is continuously cleaned via VOC/HEPA filter and UVC light. Movable chambers keep the samples safe in a stable gas and regulated temperature environment. The display indicates the patient's name being held in that chamber and allows for visual confirmation. Built-in software allows for digital verification and traceability.
The ergonomic design makes it easy to hold and carry the MIRI® M chamber, increasing the portability and safety of the embryo itself.
Limitations with Traditional Incubators
As mentioned earlier, the role of incubators in an IVF lab is very crucial as the development and safety of the embryos and gametes entirely depend upon the performance of the incubator being used.
Conventional incubators offer safe and appropriate conditions for the culture of embryos and gametes. However, for some procedures, such as fertilization (ICSI, IVF, etc.), freezing, and embryo transfer, the embryologist must remove the culture dish from this safe environment, which may compromise the safety of the sample.
Another aspect of traditional incubators that is sometimes disregarded is the necessity of shutting down the entire machine until appropriate repair and maintenance are performed if even one of the chambers fails to operate as intended. In addition to having an adverse effect on workflow, this may expose embryos to adverse circumstances.
Why Upgrade to MIRI® M?
The MIRI® M incubator has been designed specifically with the demands of a modern-day IVF lab in mind, addressing the concerns outlined above.
-
Portability and Flexibility
The ergonomic design of MIRI® M allows it to be placed wherever required. This is especially important in clinics with space constraints or where multiple procedures take place in one shared space.
-
Independent Chambers
Each MIRI® M device is made up of distinct chambers that are regulated separately. This reduces the possibility of cross-contamination by enabling the segregation of patient samples. Frequent door openings have less of an effect on the conditions of embryo culture because each chamber retains its own environment.
-
Enhanced Workflow Integration
MIRI® M has been designed to seamlessly integrate into the workflow of an IVF lab. Be it oocyte retrieval, fertilization, embryo culture, or transfer, MIRI® M can be placed and oriented for ease of access.
-
Ideal for Compact and Modular Labs
MIRI® M is well-suited for labs with limited spaces or clinics where the IVF lab may be far from the operation theatre. This ensures a high level of quality of the embryo culture even for a non-traditionally designed laboratory.
-
Stable Culture Conditions
The portability of MIRI® M means that rather than handling the culture dish outside of the incubator, the incubator itself can be carried to the destination, which reduces external exposure while handling. Furthermore, with a strict control and quick recover over temperature and gas parameters, the MIRI® M incubator features the same level of quality as its traditional counterparts.
Use Cases for the MIRI® M
- High Throughput Centres: Even in high-volume environments, the MIRI® M can be deployed as an auxiliary unit to handle overflow or specialized cultures.
- Embryo Transfer and Procedure Rooms: The ability to maintain chamber temperature for 30 minutes makes it possible to transport the culture dish holding the embryos without any exposure to outside environment.
- Clinics Prioritizing Digital Traceability: Datalogging and traceability software allow for easy tracking of the chambers and preventing mix-ups and errors.
- Labs Focused on Minimizing Human Error: Due to its ergonomic design, carrying the chamber is safer than carrying the dish itself.
Conclusion
In a time where IVF clinics grow in number and face logistical challenges like space constraints, increased caseload, etc. adoption of technological advances becomes imperative. Traditional incubators have been able to cater to the needs of a traditional setup IVF clinic, but the demands of a modern embryology lab require more adaptable, precise, and integrated systems.
The MIRI® M offers a compelling case as the next generation of embryo incubators. From its portable design to its independent multiroom chambers and improved culture stability, it presents a robust alternative to the limitations posed by conventional systems.